Liver Condition Management
ReLiV EIC project – Real Time Liver Disease Early Diagnosis Through Exhaled Volatile Organic Compounds Sensing
Metabolic dysfunction-associated steatotic liver disease (MASLD) has quietly reached epidemic levels across both adults and children.
It now affects one in three people around the world and can progress to end-stage liver disease. MASLD has become one of the main causes of chronic liver disease worldwide and is also associated with increased risk for cardiovascular morbidity, diabetes, chronic kidney disease, and other conditions.
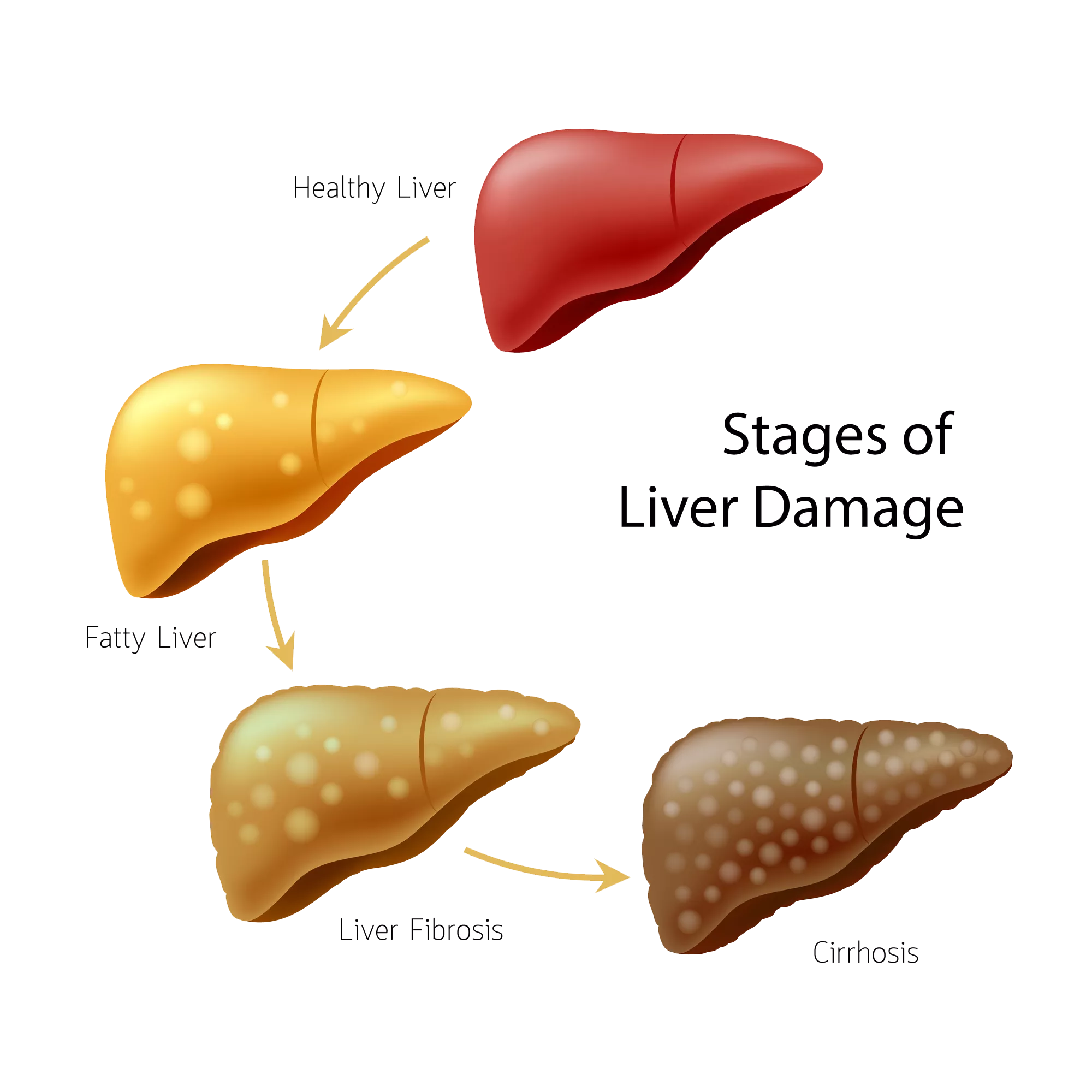
Metabolic dysfunction-associated steatohepatitis (MASH) is the aggressive form of MASLD, with liver inflammation that promotes the progressive accumulation of fibrosis in the liver parenchyma. Continuous, long-term liver scarring and damage can lead to cirrhosis. More than 20% of MASH patients could silently progress to cirrhosis over their lifetime. Cirrhosis leads to an increased risk of developing a type of liver cancer called hepatocellular carcinoma (HCC), as well as liver failure and, unless a transplant is performed, significant morbidity and death.
The prevalence of MASH is projected to double by 2030; accordingly, the incidence of hepatic decompensation, hepatocellular carcinoma, and NASH-cirrhosis-related mortality is estimated to increase two- to three-fold.
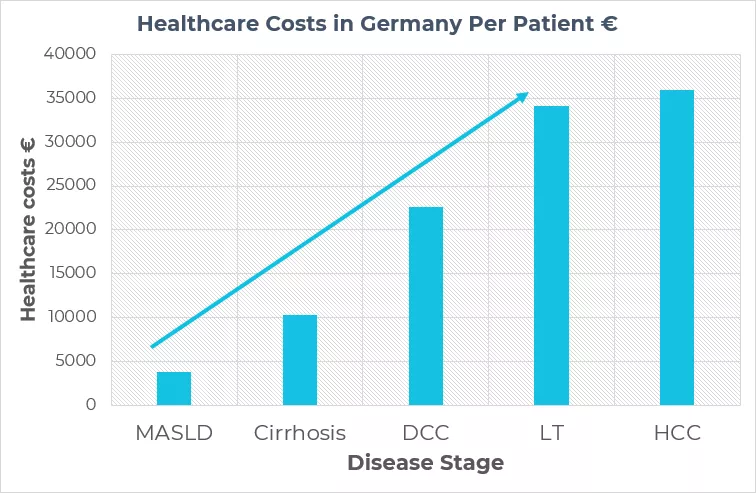
The burden of advanced liver disease due to MASLD will more than double during 2016-2030, and the annual predicted economic burden of MASLD in Europe will be more than €35 billion in direct costs and a further €200 billion in societal cost. To learn more: click here
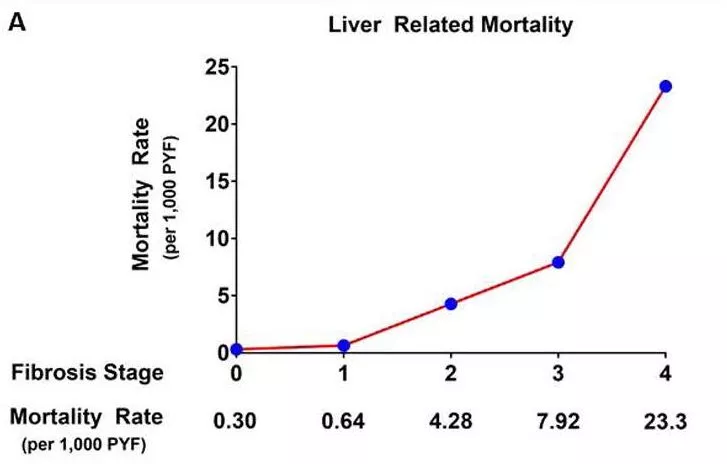
According to a NIH study, the annual cost per patient in Germany increases dramatically with the stage of the disease:
Cirrhosis increases mortality rates by ~400%
MASH is a silent disease and diagnosis before complications occur is still a challenge.
MASH generally has no, or only a few, non-specific symptoms until very late stage and thus diagnosis before the occurrence of complications is a challenge for physicians.
With care, the liver can heal itself, but without timely action liver damage can become permanent and contribute to increasing trends in MASLD-related morbidity and mortality.
MASLD’s high and increasing prevalence and associated personal, societal, and healthcare burden emphasize the need for early detection when lifestyle changes and therapeutics can be effectively deployed to reduce the number of patients with progressive disease who are experiencing severe symptoms.
Current diagnosis
The majority of patients with MASLD are followed up in the community by general practitioners (GPs).
A diagnosis can be made through physical exams, blood tests and imaging tests (such as an ultrasound); however, all of them are limited in their ability to diagnose patients with significant/advanced fibrosis. For definite diagnosis, a liver biopsy is required, but this is associated with several limitations including its invasiveness and dangerous complications.
As a result, patients with advanced fibrosis or cirrhosis who could benefit from specialist interventions often remain undetected until they present with cirrhosis and severe complications including hepatocellular carcinoma.
This ineffective management contributes to the poor outcomes associated with liver disease and the increasing trends in MASLD-related morbidity and mortality.
The FDA just recently published a paper about the immense need for non-invasive tests and to accurately classify the stage of fibrosis. Find out more about it click here
DiaNose solution – A non-Invasive breath test
The liver is highly metabolically active, making it a prime source of volatile organic compounds (VOCs) in the breath.
Thus a non-invasive breath test provides a preferable and reliable approach for liver disease detection.
With the DiaNose breath test, the healthcare system will have an innovative, cost-effective tool to identify liver disease earlier and provide timely specialist care to patients.
With the significant support of the EIC-Transition grant and our innovative approach, we expect to bring the first non-invasive, user-friendly, and widely-accessible MASH/cirrhosis test to the market
European Innovation Council program under grant agreement 101158688